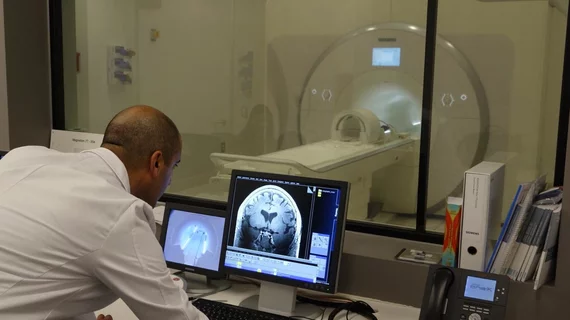
The FDA recently approved the first ultra-high-field 7T Terra MRI scanner in North America for clinical use, according to the University of Southern California (USC).
The scanner—installed at USC’s Mark and Mary Stevens Neuroimaging and Informatics Institute (INI) in February 2017—may help in the development of care, treatment and monitoring of patients with Alzheimer’s disease, multiple sclerosis and other neurological diseases.
"This device, which has already made its mark as a powerful tool to advance research in the neurosciences, is now accessible to clinical populations in addition to researchers," Arthur W. Toga, PhD, director of the Stevens INI and chair in neuroscience at USC’s Keck School of Medicine, said in a prepared statement. "Clinicians across the university and beyond can now leverage all the benefits of increased spatial resolution to serve patients in need."
With a magnetic field of a 7 Tesla, the scanner possesses a strength four-times greater than standard MRIs and has a stronger signal-to-noise ratio. Both components allow researchers and clinicians to collect neuroimages with higher resolution, contrast and detail.
"Ideally, we want to look at the smallest group of neurons possible, so we can start to pinpoint what's happening at the cellular level,” said Danny JJ Wang, PhD, director of imaging technology innovation at the INI. "The 7T may save patients an invasive procedure. It also makes it easier for neurosurgeons to selectively remove a tumor without damaging surrounding areas.”