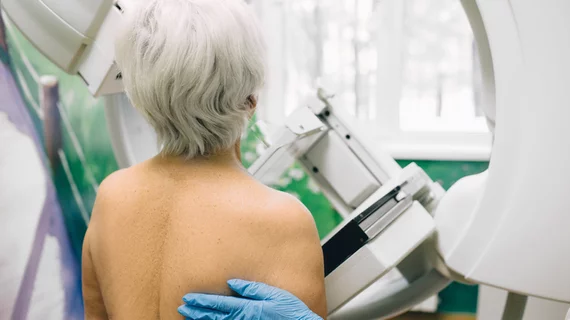
The U.S. Food & Drug Administration issued another imaging alert this morning, warning patients who received mammograms at a women’s imaging center located in eastern New Jersey.
Advanced Women’s Imaging failed to perform required quality control tests between March 11 and August 25, 2020, the FDA discovered during the facility’s annual Mammography Quality Standards inspection.
The American College of Radiology then requested the Guttenberg, New Jersey, center provide clinical images and documentation to ensure its mammography standards were not compromised. Advanced Women’s Imaging, however, did not comply and on Sept. 12, the organization’s accreditation expired.
Three days later, the FDA alerted the imaging center that it was no longer certified and must immediately stop performing mammography exams.
“The FDA will continue to monitor this issue and keep the public informed as new information becomes available,” the administration said in its May 21 update. “At this time, the FDA recommends that patents contact Advanced Women Imaging to gain access to their medical records.”
Just yesterday, May 20, the federal organization dedicated to public health issued a similar warning to patients who received a mammography exam at Laurel Radiology Services in Laurel, Maryland. That organization’s annual review turned up a litany of quality issues, including some “severe” deficiencies.