EchoNous, the Seattle-based medical tool provider, recently received FDA clearance for the its ultrasound-based tool designed for nurses to improve peripheral IV (PIV) catheter placements.
“We designed the EchoNous Vein to provide nurses with rapid, clear images of veins to directly improve patient care, satisfaction and HCAHPS scores,” said Kevin Goodwin, CEO of EchoNous, in a prepared statement. “We wanted to create an easy-to-use tool that would help to reduce failed sticks, as with each failure comes a much higher risk of vessel trauma or infections which can lead to longer stays in hospital and higher medical costs.”
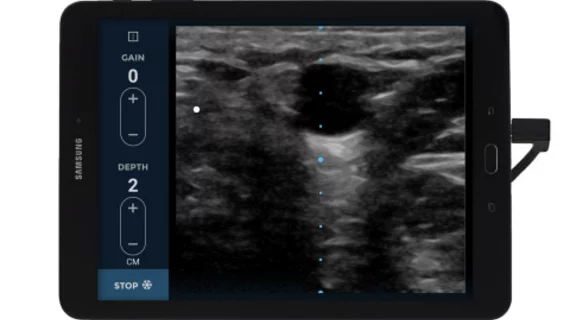
The tool can provide clear images of a patient's veins at a depth of one to five centimeters with the control of two buttons, which is critical when patients need an infusion or antibiotic treatment in a timely manner, according to an EchoNous press release.
Through on-screen controls, clinicians using ultrasound can clearly identify veins in the center of the display to locate veins and evaluate health and quality.
“As clinicians we know that IV insertion and selecting a healthy vein for catheter placement can help to reduce the chance of post insertional complications," said Nancy Moureau, PhD, CEO of PICC Excellence, in a prepared statement "To have a tool specifically designed to easily select veins and guide catheter placement is extremely valuable as we’re always looking to improve the patient experience.”